US scientists created a novel tool to fine-tune catalyst qualities. By adjusting the voltage, the ‘catalytic condenser’ can make inexpensive metals act like more costly ones. With the new technology, it’s feasible to circumvent each catalyst’s’speed limit,’ creating new prospects in energy research and other disciplines.
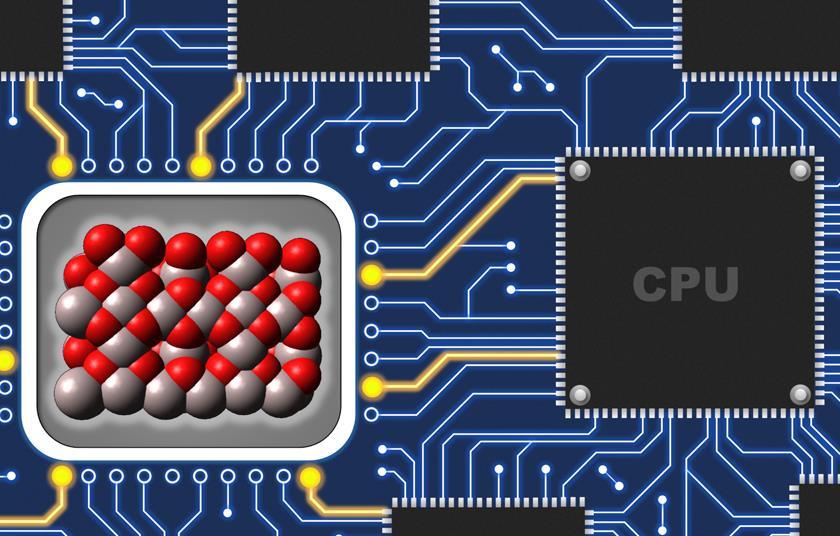
The catalytic condenser collects electrons or holes in a catalyst film, changing its electrical and catalytic characteristics. We can modify the catalyst as rapidly as the chemistry, he explains.
Static metals and oxides are traditional catalysts. Dauenhauer: ‘They stay the same for months and years while surface responses occurs.’ Catalysts have speed and selectivity restrictions. Until previously, essential reactions like generating hydrogen from water or synthesising ammonia were constrained by the Sabatier principle, which dictates that a catalyst’s interactions with a substrate must be exact. We have materials that reach this speed limit, but the reactions are too slow for technology needed to address climate change.
The novel catalytic condenser adds and removes electrons from surfaces quickly. Dauenhauer says, ‘Catalysts may now be swiftly designed by electronic dials, rather than expensive, sluggish, iterative synthesis, characterization, and testing.’ It’s a breakthrough in catalyst research.
It comprises four parts. Starting with a silicon wafer, the researchers developed an electrically insulating hafnium oxide layer, then a conductive graphene sheet, and lastly the catalyst, an alumina film. By providing a voltage differential between the conductive silicon wafer and graphene/catalyst layer, electrons and holes accumulate. When there are enough electrons or holes in the catalyst layer, the material becomes catalytic,’ says Dauenhauer. In the alumina-on-graphene catalytic condenser, around 12% of an electron was taken from the catalyst for every area where chemistry might occur.
The device draws on Dauenhauer and colleagues’ 2019 catalytic resonance theory, which predicts rates considerably above the Sabatier limit if the catalyst may vary over time. Dauenhauer claims the present gadget can work at 1000 hertz because graphene’s electrons travel swiftly. As a platform device, the condenser can have an active surface of any metal and hundreds of potential metal oxides, allowing dynamic catalyst operation for thousands of chemical industrial applications. Computational simulations of the ammonia production process have revealed this.
Changing one metal to function like another is useful when utilising inexpensive, less active and less selective catalysts, explains Kandis Leslie Gilliard-AbdulAziz of the University of California, Riverside. This is a problem when replacing pricey precious metal catalysts with nickel, iron, or cobalt.
Programmable catalytic condenser, says Dauenhauer. ‘A sinusoidal voltage oscillation can switch a catalyst between two states, but more complicated programmes are feasible. Future condensers and algorithms will be optimised for enhanced chemical control.

Be First to Comment